Chemistry
Chemistry
4665872 / 5558097
Noble Gases
A Family of Noble Disposition
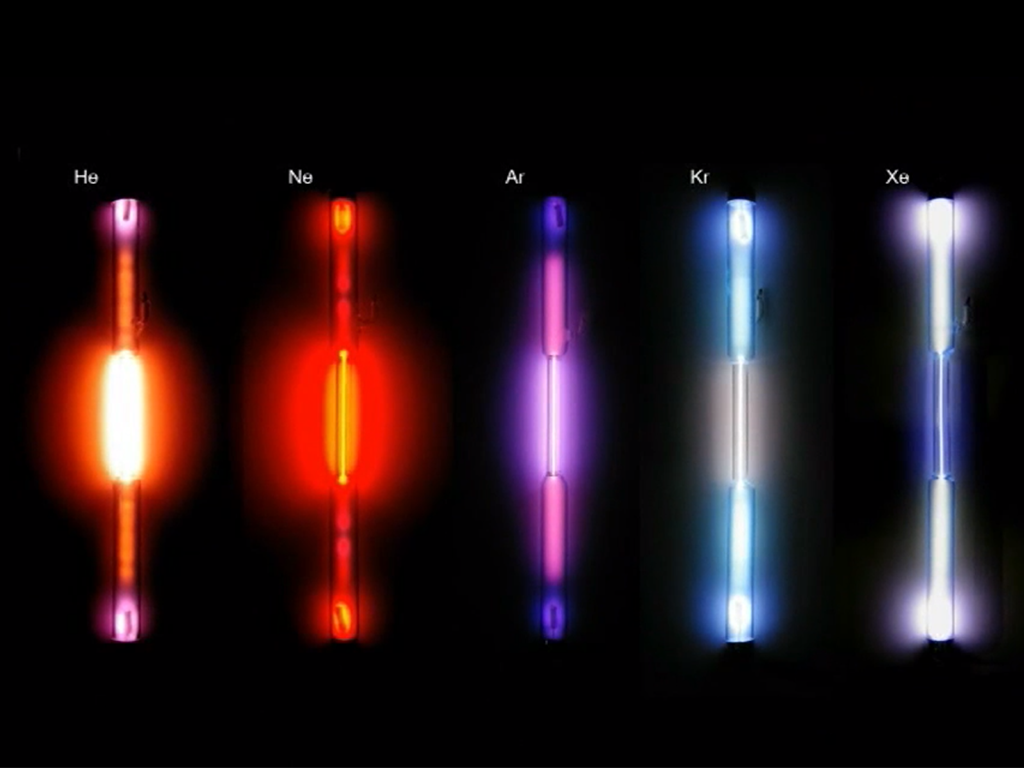
Xenon, Helium, Neon, Argon, Krypton and the radioactive Radon belong to the noble gases. These form the family of noble gases as the elements of the eighth group of the periodic table. All of them are colourless and odourless, non-inflammable and non-toxic. Their most striking chemical property is their inertness. This can be explained by their electron arrangement, termed noble gas configuration and represents a particularly stable and therefore low-energy state. The noble gases are to be found in scant amounts in our air from which they are also distilled. Helium is mainly extracted from natural gas. In everyday life, we encounter noble gases for example as shielding, filling or buoyant gases and in fluorescent tubes. The shell model describes the structure of the atoms. It is based on the distribution of electrons in restricted areas at a fixed distance around the core of the atom.
Play trailer
Curriculum-centred and oriented towards educational standards
Matching
Air Traffic
Being able to fly has been a dream of humanity from time immemorial. But it does not even date back a century that people actually started being able to travel through the air. Since the 1960s, the number of flight passengers has been constantly increasing. Thus, the airspace is no longer dominated by birds but by man-made flying objects.
Stalking
n Germany, 12 % of all federal citizens are pursued by a stalker once in their lives. And not only celebrities are among their victims! Everyone may be confronted with such a situation.